
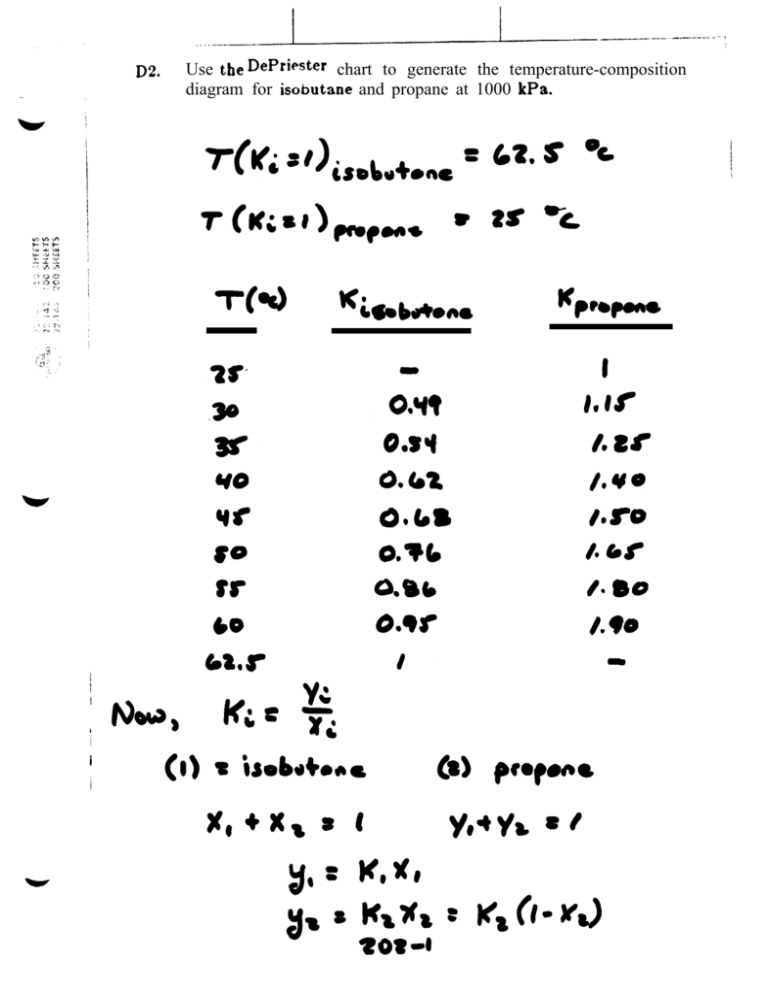
#K value depriester chart pdf#
Once you have a solution to the exercises you will submit your answers as a PDF by uploading your file to be graded. The rest should be type-written for ease of reading when grading. The scanned pages should relate to using the graphs for solving the problem. Important Note: You may submit scanned images or clear handwritten pages as a PDF that is less than 2 MB in size for this exercise. Submit your answers as a PDF in the Exercise 4 assignment inside the Lesson 5 Module, showing all the steps in your calculations, indicate the K values you read from the nomograms, and state your assumptions, if any. Would the summer, or winter asphalt product be “heavier”? Explain why. phase mole fraction divided by the liquid phase mole fraction, known as the. Explain which of the following switches will take place in the refinery in about a month, and why? 40 ptsĪ) Switch the deasphalting solvent from propane to pentane.ī) Switch the deasphalting solvent from pentane to propane. In late fall this refinery switches operations to produce more fuel oil from VDR for the coming winter months, producing still some asphalt, but in lower quantity. The average absolute error between experimental and predicted K-values for the new model was 4.355% compared to 20.5% for the Almehaideb correlation, 76.1% for the Whitson and Torp correlation, 84.27% for the Wilson correlation, and 105.8 for the McWilliams correlation.A refinery in Northwest Pennsylvania produces asphalt as an important product that brings revenue, particularly during the summer months.

Comparisons of results show that the currently published correlations give poor estimates of K-values for all components, while the proposed new model improved significantly the average absolute deviation error for all components. These K-values were then used to build the model using the Discipulus software, a commercial Genetic Programming system, and the results of K-values were compared with the values obtained from published correlations. McWilliams ( McWilliams, 1973 ) fitted these charts to the following polynomial equation (5) ln K a T 1 T 2 + a T 2 T + a T 3 + a p 1 ln p + a p 2 p 2 + a p 3 p where T in R and p. pressure and temperature that are valid up to around 6000 psi pressures or more. Material balance techniques were used to extract the K-values of crude oil and gas components from the constant volume depletion and differential liberation tests for the oil and gas samples, respectively. DePriester (DePriester, 1953) presented K-value charts for light hydrocarbons vs. Constant Volume Depletion (CVD) and Differential Liberation (DL) were conducted for these samples. In this paper, 732 high-pressure K-values obtained from PVT analysis of 17 crude oil and gas samples from a number of petroleum reservoirs in Arabian Gulf are used. The new model is applied to multicomponent mixtures. This paper presents a new model for predicting K values with genetic programming (GP). of mercury, which it will support at a temperature of 273 K in a standard. Several techniques are available in the literature to estimate the K-values. chemical reaction conversions above the equilibrium value could never be. Our new CrystalGraphics Chart and Diagram Slides for PowerPoint is a collection of over 1000 impressively designed data-driven chart and editable diagram s guaranteed to impress any audience.
In particular, they are critical for reliable and successful compositional reservoir simulation. Chart and Diagram Slides for PowerPoint - Beautifully designed chart and diagram s for PowerPoint with visually stunning graphics and animation effects. They are important in predicting compositional changes under varying temperatures and pressures in the reservoirs, surface separators, and production and transportation facilities. K (or DePriester) Chart (high T range) in American Engineering Units from Introduction to Chemical Engineering Thermodynamics (7th ed) by Smith, J.M., Van Ness, H.C., and Abbott, M.M. Equilibrium ratios play a fundamental role in understanding the phase behavior of hydrocarbon mixtures.


 0 kommentar(er)
0 kommentar(er)
